tin electron configuration|sn ground state electron configuration : Clark Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (II) compounds with its two unpaired p-electrons. In the three dimensional figure below, the first and most inner . What Are the Best Coordinating Colors For SW Perfect Greige? . Perfect Greige vs. Mega Greige. These greiges are pretty much alike with Mega being somewhat warmer and, well, deeper in tones with more prominent beige (some would even say brownish) undertones. However, due to such a similarity, they are not a good color .
tin electron configuration,Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (II) compounds with its two unpaired p-electrons. In the three dimensional figure below, the first and most inner .Learn how to write the complete electron configuration of tin (Sn) using the orbit and orbital models. Compare the differences and similarities between the tw.Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. .
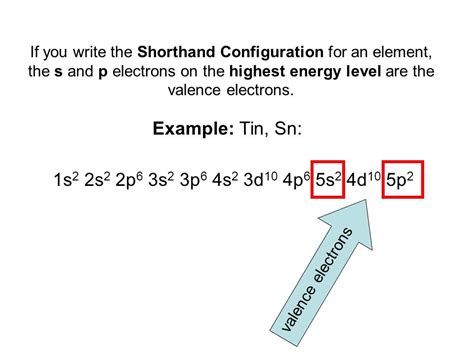
tin electron configuration sn ground state electron configuration Mar 23, 2023 The Electron configuration of Tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Tin is the chemical element of the periodic table which is in group 14, its atomic number is .tin electron configurationTin is a post-transition metal with atomic number 50 and chemical symbol Sn. Its electron configuration is [Kr] 4d 10 5s 2 5p 2, with valence electrons 4 and valency electrons 2,4. The tin electron configuration, represented as 5s 2 4d 10 5p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2, illustrates the precise arrangement of electrons within the atom.

Tin Electron Configuration. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4.sn ground state electron configuration How to write Tin electron configuration. The electronic configuration of the Tin is 1s 2, 2s 2, 2p 6, 3s 2, 3p 6, 4s 2, 3d 10, 4p 6, 5s 2, and 4d 10, 5p 2 and is written . Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us .To check the answer, verify that the subscripts add up to the atomic number. In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron . Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic structure.The chemical symbol for Tin is Sn. Electron Configuration and Oxidation States of Tin. Electron configuration of Tin is [Kr] 4d10 5s2 5p2. Possible oxidation states are +2,4. Electron Configuration. The .Orbital diagram. Tin electron configuration. ← Electronic configurations of elements. Sn (Tin) is an element with position number 50 in the periodic table. Located in the V period. Melting point: 232 ℃. Density: 7.29 g/cm 3 . Electronic configuration of the Tin atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 .Electron Configuration of Tin. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (II) compounds with its two unpaired p-electrons. In the three dimensional figure below, the first and most inner electron shell is represented by blue electrons, the second electron . The electron configuration of tin is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. Learn how to find: Tin electron configuration. Now in the next step, start drawing the orbital diagram for tin. Draw orbital diagram. Before drawing the orbital diagram, you should know the three general rules. Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full. . Electron configuration of Tin (Sn) [Kr] 4d 10 5s 2 5p 2: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2: 2, 8, 18, 18, 4: 51:

Tin. Full electron configuration of tin: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. indium ← tin → antimony. Tin, complete electron configuration.
tin electron configuration|sn ground state electron configuration
PH0 · what is zincs electron configuration
PH1 · tin electron configuration full
PH2 · sn ground state electron configuration
PH3 · full electron configuration of platinum
PH4 · electron configuration for every element
PH5 · electron configuration chart
PH6 · electron configuration calculator
PH7 · abbreviated electron configuration for tellurium
PH8 · Iba pa